What can we do to help clean unhealthy water?
David Reckhow is an engineer at the University of Massachusetts Amherst. A large shed on the outskirts of town has become his world-class laboratory. That's why many experts want to use this lab — more than the building has space for. These people want to test their new technologies to clean up drinking water.
To tackle the lab's popularity, he'll roll out a new annex next year — one on wheels. This Mobile Water Innovation Laboratory will take promising new and affordable technologies to local communities for testing.

David Reckhow and his colleagues at UMass Amherst turned an old building into a new lab. They use it to test the latest technologies to treat drinking water.
David Reckhow
U.S. drinking water is heavily regulated. Overall, it also tends to be pretty clean. Still, several recent water-poisoning cases have grabbed national headlines. Probably the most well-known was the 2014 lead crisis in Flint, Mich. Lead is a toxic, heavy metal that has been used in many water pipes around the nation. Studies have shown it can sicken people and permanently lower a developing child's IQ. A change in how Flint's drinking water was treated exposed an estimated 99,000 city residents — many of them children — to elevated levels of lead.
Educators and Parents, Sign Up for The Cheat Sheet
Weekly updates to help you use Science News for Students in the learning environment
Thank you for signing up!
There was a problem signing you up.
Such events pointed to ongoing weaknesses in how some water is treated. It also shook up the trust of many people in their tap water.
Flint was not an isolated water crisis. In just the 2013 to 2014 period, 42 outbreaks of drinking water poisonings were recorded by the U.S. Centers for Disease Control and Prevention, or CDC. These outbreaks led to more than 1,000 people getting sick. Thirteen died. The top culprits were Legionella bacteria and some form of chemical, toxin or parasite. CDC reported these data in the November 10, 2017 Morbidity & Mortality Weekly Report.
Six things that shouldn't pollute your drinking water
But such numbers tell only part of the story. Many pollutants that the U.S. Environmental Protection Agency regulates cause problems only when people are exposed for months to years. For instance, the effects of lead don't tend to show up right away. One study last February surveyed records from 1982 to 2015 of drinking water that didn't meet EPA's standards. (Those were the most recent years for which data were available.) It found that 21 million people were served by drinking water systems that failed U.S. standards. Researchers reported their findings in the Proceedings of the National Academy of Sciences.
All of this helps explain why there is so much interest in ways to better disinfect drinking water, filter out poisons and detect when water-treatment slipups took place.
Explainer: How is water cleaned up for drinking
Current technology can remove most pollutants, says David Sedlak. He's an environmental engineer who works at the University of California, Berkeley. Those pollutants include microbes, arsenic, nitrates and lead. He points to others, however, "that are very difficult to degrade or transform." These include industrial chemicals, such as the ones used to make water- and stain-repellent treatments for fabrics and more.
Smaller communities, especially, can't always afford top-of-the-line equipment to pull out challenging pollutants. Many also can't afford to replace leaking or lead-based pipes. So Reckhow's facility is testing new, more affordable approaches to help such communities.
Some researchers are adding technologies to deal with new and potentially harmful pollutants. Others are designing approaches that work with existing water systems. Still others aim to clean up pollutants at their source.

The Mobile Water Innovation Laboratory (left) is a trailer that will test new drinking water technologies around Massachusetts. Inside the van (right) is a flexible setup of filters, pipes and chemicals that are used in testing.
John Solem/UMass Amherst
New tech solutions
Reckhow's team at UMass Amherst is testing ferrate as a replacement for several water treatment steps. As an electrically charged form of iron, ferrate is an ion. This material kills bacteria in the water. But it also had an added benefit. It breaks down carbon-based pollutants into less harmful chemicals.
Finally, ferrate makes ions of the metal manganese less soluble in water. That will make them easier to filter out, say Reckhow and his colleagues. They described the treatment in a 2016 paper in Journal–American Water Association.
With its many benefits, ferrate might help streamline the drinking-water treatment, says Joseph Goodwill. He's an environmental engineer who works at the University of Rhode Island in Kingston. Ferrate might also cut the need for disinfectants. Some of these, such as chlorine, can yield dangerous by-products, he notes.
Some water-treatment plants use ozone gas to break down pollutants. But it's costly. Ferrate should cost less, making it attractive to smaller water-cleanup plants, Reckhow says. Early next year, his mobile water treatment lab plans to test ferrate water treatment in the small town of Gloucester, Mass.
Brian Chaplin is an engineer at the University of Illinois at Chicago. He notes that some water-filtering membranes can get clogged with small particles. Unclogging the filter wastes energy. It also ups the cost of treating water. Electricity might solve that problem, Chaplin suggests, and offer some side benefits.
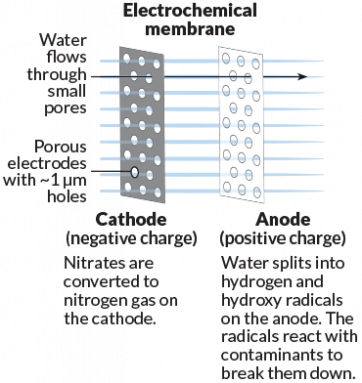
Like a traditional membrane, this electrochemical membrane filters out contaminants by size. An added benefit: It can break down contaminants via chemical reactions on the membrane's surface.
Art: E. Otwell; Source: B. Chaplin
His team tested a special electrically charged membrane made from titanium oxide or titanium dioxide. This electrochemical membrane not only filters water but also acts as an electrode. Chemical reactions happening on such a charged membrane can turn nitrates — a pollutant — into nitrogen gas. Or the membrane might split water molecules, generating reactive ions that can kill infectious microbes in the water. The reactions also prevent particles from sticking to the membrane. Large carbon-based chemicals, such as benzene, now become smaller and less harmful.
In lab tests, these new membranes were successful in filtering out and destroying pollutants, Chaplin says. In one test, a membrane transformed 67 percent of the nitrates into other molecules. The finished water was below the EPA's regulatory limit for nitrate of 10 parts per million. He and colleagues reported their results last July in Environmental Science and Technology. Chaplin expects to move the membrane into pilot tests within the next two years.
Industrial chemicals known as PFAs present two challenges. Only the larger ones are effectively removed by activated carbon, the filtering substance in many household water filters. Smaller molecules will remain in the water, notes Christopher Higgins. He's an environmental engineer at the Colorado School of Mines (CSM) in Golden. What's more, filtering isn't a simple solution for these pollutants. After all, once removed, they still are hard to break down for safe disposal.
Scientists Say: Runoff
So he and his CSM colleague Timothy Strathmann are working on a process to destroy PFAs. First, they use a specialized filter with tiny holes to grab the molecules out of the water. Then they add sulfite to the concentrated mix of PFAs. When later hit with ultraviolet light, the sulfite generates reactive electrons. These break down the tough carbon-fluorine bonds in the PFA molecules. Within 30 minutes, the UV-sulfites combo almost completely destroyed one type of PFA chemical.
Soon, Higgins and Strathmann will test the process at Peterson Air Force Base in Colorado. It's one of nearly 200 U.S. sites known to have groundwater tainted by PFAs. Cleaning up those sites would remove the pollutants before they could be used to feed wells or city water systems.
Power Words
More About Power Wordsanode The negative terminal of a battery, and the positively charged electrode in an electrolytic cell. It attracts negatively charged particles. The anode is the source of electrons for use outside the battery when it discharges.
arsenic A highly poisonous metallic element. It occurs in three chemically different forms, which also vary by color (yellow, black and gray). The brittle, crystalline (gray) form is the most common. Some manufacturers tap its toxicity by adding it to insecticides.
bacteria (singular: bacterium) Single-celled organisms. These dwell nearly everywhere on Earth, from the bottom of the sea to inside other living organisms (such as plants and animals).
benzene A ring-shaped hydrocarbon molecule made from six carbon and six hydrogen atoms. It's liquid at room temperature and easily evaporates into the air. It's widely used in industry and a natural constituent of petroleum, gasoline and cigarette smoke. It is highly toxic if breathed in large amounts and may cause cancer after prolonged, lower dose exposure.
bond (in chemistry) A semi-permanent attachment between atoms — or groups of atoms — in a molecule. It's formed by an attractive force between the participating atoms. Once bonded, the atoms will work as a unit. To separate the component atoms, energy must be supplied to the molecule as heat or some other type of radiation.
carbon The chemical element having the atomic number 6. It is the physical basis of all life on Earth. Carbon exists freely as graphite and diamond. It is an important part of coal, limestone and petroleum, and is capable of self-bonding, chemically, to form an enormous number of chemically, biologically and commercially important molecules.
cathode The positive terminal of a battery, and the negatively charged electrode in an electrolytic cell. It attracts positively charged particles. During discharge, the cathode attracts electrons from outside the battery.
Centers for Disease Control and Prevention, or CDC An agency of the U.S. Department of Health and Human Services, CDC is charged with protecting public health and safety by working to control and prevent disease, injury and disabilities. It does this by investigating disease outbreaks, tracking exposures by Americans to infections and toxic chemicals, and regularly surveying diet and other habits among a representative cross-section of all Americans.
chemical A substance formed from two or more atoms that unite (bond) in a fixed proportion and structure. For example, water is a chemical made when two hydrogen atoms bond to one oxygen atom. Its chemical formula is H2O. Chemical also can be an adjective to describe properties of materials that are the result of various reactions between different compounds.
chemical reaction A process that involves the rearrangement of the molecules or structure of a substance, as opposed to a change in physical form (as from a solid to a gas).
chlorine A chemical element with the scientific symbol Cl. It is sometimes used to kill germs in water. Compounds that contain chlorine are called chlorides.
colleague Someone who works with another; a co-worker or team member.
degrade To break down into smaller, simpler materials — as when wood rots or as a flag that's left outdoors in the weather will fray, fade and fall apart. (in chemistry) To break down a compound into smaller components.
disinfect To clean an area by killing dangerous infectious organisms, such as disease-causing bacteria.
electricity A flow of charge, usually from the movement of negatively charged particles, called electrons.
electrode A device that conducts electricity and is used to make contact with non-metal part of an electrical circuit, or that contacts something through which an electrical signal moves.
electron A negatively charged particle, usually found orbiting the outer regions of an atom; also, the carrier of electricity within solids.
environmental engineer A person who uses science to study and solve problems in ecosystems — from forests to the human body.
Environmental Protection Agency (or EPA) A national government agency charged with helping create a cleaner, safer and healthier environment in the United States. Created on Dec. 2, 1970, it reviews data on the possible toxicity of new chemicals (other than foods or drugs, which are regulated by other agencies) before they are approved for sale and use. Where such chemicals may be toxic, it sets limits or guidelines on how much of them may be released into (or allowed to build up in) the air, water or soil.
environmental science The study of ecosystems to help identify environmental problems and possible solutions. Environmental science can bring together many fields including physics, chemistry, biology and oceanography to understand how ecosystems function and how humans can coexist with them in harmony. People who work in this field are known as environmental scientists.
filter (in chemistry and environmental science) A device or system that allows some materials to pass through but not others, based on their size or some other feature.
fluorine An element first discovered in 1886 by Henri Moissan. It takes its name from the Latin word meaning "to flow." Very reactive, chemically, this element had little commercial use until World War II, when it was used to help make a nuclear-reactor fuel. Later, it was used as ingredients (fluorocarbons) in refrigerants and aerosol propellants. Most recently, it has found widespread use to make nonstick coatings for frying pans, plumbers' tape, and waterproof clothing.
force Some outside influence that can change the motion of a body, hold bodies close to one another, or produce motion or stress in a stationary body.
groundwater Water that is held underground in the soil or in pores and crevices in rock.
infectious An adjective that describes a type of germ that can be transmitted to people, animals or other living things.
innovation (v. to innovate; adj. innovative) An adaptation or improvement to an existing idea, process or product that is new, clever, more effective or more practical.
ion (adj. ionized) An atom or molecule with an electric charge due to the loss or gain of one or more electrons. An ionized gas, or plasma, is where all of the electrons have been separated from their parent atoms.
IQ Short for intelligence quotient. It's a number representing a person's reasoning ability. It's determined by dividing a person's score on a special test by his or her age, then multiplying by 100.
iron A metallic element that is common within minerals in Earth's crust and in its hot core. This metal also is found in cosmic dust and in many meteorites.
lead A toxic heavy metal (abbreviated as Pb) that in the body moves to where calcium wants to go (such as bones and teeth). The metal is particularly toxic to the brain. In a child's developing brain, it can permanently impair IQ, even at relatively low levels.
manganese Chemical element with the atomic number 25. It's a hard gray metal in the transition series. Manganese is an important component of special steels.
mass A number that shows how much an object resists speeding up and slowing down — basically a measure of how much matter that object is made from.
membrane A barrier which blocks the passage (or flow through) of some materials depending on their size or other features. Membranes are an integral part of filtration systems. Many serve that same function as the outer covering of cells or organs of a body.
metal Something that conducts electricity well, tends to be shiny (reflective) and malleable (meaning it can be reshaped with heat and not too much force or pressure).
microbe Short for microorganism. A living thing that is too small to see with the unaided eye, including bacteria, some fungi and many other organisms such as amoebas. Most consist of a single cell.
molecule An electrically neutral group of atoms that represents the smallest possible amount of a chemical compound. Molecules can be made of single types of atoms or of different types. For example, the oxygen in the air is made of two oxygen atoms (O2), but water is made of two hydrogen atoms and one oxygen atom (H2O).
morbidity The prevalence of illness; the share of people having a particular sickness at some particular time or in some particular place.
mortality Deaths. From mortal, meaning deadly.
nitrate An ion formed by the combination of a nitrogen atom bound to three oxygen atoms. The term is also used as a general name for any of various related compounds formed by the combination of such atoms.
nitrogen A colorless, odorless and nonreactive gaseous element that forms about 78 percent of Earth's atmosphere. Its scientific symbol is N. Nitrogen is released in the form of nitrogen oxides as fossil fuels burn.
outbreak The sudden emergence of disease in a population of people or animals. The term may also be applied to the sudden emergence of devastating natural phenomena, such as earthquakes or tornadoes.
oxide A compound made by combining one or more elements with oxygen. Rust is an oxide; so is water.
ozone A colorless gas that forms high in the atmosphere and at ground level. When it forms at Earth's surface, ozone is a pollutant that irritates eyes and lungs. It is also a major ingredient of smog. It can also be used as a disinfectant as it kills cells, including microbes.
parasite An organism that gets benefits from another species, called a host, but doesn't provide that host any benefits. Classic examples of parasites include ticks, fleas and tapeworms.
particle A minute amount of something.
pollutant A substance that taints something — such as the air, water, our bodies or products. Some pollutants are chemicals, such as pesticides. Others may be radiation, including excess heat or light. Even weeds and other invasive species can be considered a type of biological pollution.
Proceedings of the National Academy of Sciences A prestigious journal publishing original scientific research, begun in 1914. The journal's content spans the biological, physical, and social sciences. Each of the more than 3,000 papers it publishes each year, now, are not only peer reviewed but also approved by a member of the U.S. National Academy of Sciences.
radical An charged molecule having one or more unpaired outer electrons. Radicals readily take part in chemical reactions. The body is capable of making radicals as one means to kill cells, and thereby rid itself of damaged cells or infectious microbes.
reactive (in chemistry) The tendency of a substance to take part in a chemical process, known as a reaction, that leads to new chemicals or changes in existing chemicals.
regulate (n. regulation) To control with actions. Governments write rules and regulations — laws — that are enforced by police and the courts.
resident Some member of a community of organisms that lives in a particular place. (Antonym: visitor)
soluble Some chemical that is able to dissolve some liquid. The resulting combo becomes a solution.
solution A liquid in which one chemical has been dissolved into another.
standards (in regulations) A limit above which something may not be used, sold or considered safe.
technology The application of scientific knowledge for practical purposes, especially in industry — or the devices, processes and systems that result from those efforts.
titanium dioxide A white, unreactive, solid material that occurs naturally as a mineral and is used extensively as a white pigment.
toxic Poisonous or able to harm or kill cells, tissues or whole organisms. The measure of risk posed by such a poison is its toxicity.
toxin A poison produced by living organisms, such as germs, bees, spiders, poison ivy and snakes.
ultraviolet light A type of electromagnetic radiation with a wavelength from 10 nanometers to 380 nanometers. The wavelengths are shorter than that of visible light but longer than X-rays.
waste Any materials that are left over from biological or other systems that have no value, so they can be disposed of as trash or recycled for some new use.
Citations
Journal: P. Gayen et al. Electrocatalytic reduction of nitrate using Magnéli phase TiO2 reactive electrochemical membranes doped with Pd-based catalysts. Environmental Science & Technology. Vol. 52, August 21, 2018, p. 9370. doi: 10.1021/acs.est.8b03038.
Report: B. Mostafavi. Study examines blood lead levels of Flint children. University of Michigan Health Lab. March 26, 2018.
Journal: M. Allaire et al. National trends in drinking water quality. Proceedings of the National Academy of Sciences. Vol. 115, February 27, 2018, p. 2078. doi: 10.1073/pnas.1719805115.
Report: K.M. Benedict et al. Surveillance for waterborne disease outbreaks associated with drinking water — United States, 2013–2014. Morbidity & Mortality Weekly Report. Vol. 66, November 10, 2017, p. 1216.
Report: C. Kennedy et al. Blood lead levels among children aged <6 years — Flint, Michigan, 2013–2016. Morbidity & Mortality Weekly Report. Vol. 65, July 1, 2016.
Journal: J.E. Goodwill et al. Laboratory assessment of ferrate for drinking water treatment. Journal-American Water Association. Vol. 108, March 1, 2016, p. E-164. doi: 10.5942/jawwa.2016.108.0029.
Journal: M. Hanna-Attisha et al. Elevated blood lead levels in children associated with the Flint drinking water crisis: A spatial analysis of risk and public health response. American Journal of Public Health. Vol. 106, February 2016, p. 283. doi: 10.2105/AJPH.2015.303003.
Journal: M. Edwards. Fetal death and reduced birth rates associated with exposure to lead-contaminated drinking water. Environmental Science & Technology. Vol. 48, January 7, 2014, p. 739. doi: 10.1021/es4034952.
Webpage: EPA. Reducing PFAS in drinking water with treatment technologies. August 23, 2018.
Learn more about the UMass mobile testing lab.
What can we do to help clean unhealthy water?
Source: https://www.sciencenewsforstudents.org/article/new-ways-clean-polluted-sources-drinking-water
Posted by: eagletromsented.blogspot.com

0 Response to "What can we do to help clean unhealthy water?"
Post a Comment